Unbiunium
अनबीउनियम, जिसे एका-एक्टिनियम या तत्व 121 के नाम से भी जाना जाता है, एक अनुमानित रासायनिक तत्व है जिसका परमाणु संख्या 121 है। यह एक सिस्टमैटिक नाम है जो इस तत्व के पता चलने, पुष्टिकरण और एक स्थायी नाम के निर्धारण तक उपयोग किया जाता है। इसका रासायनिक प्रतीक Ubu है। परिकल्पित आवर्त सारणी में, यह सुपरएक्टिनाइड के रूप में जाना जाता है, जो आवर्त सारणी के आठवें अवधि का तीसरा तत्व है। इसे बहुत अधिक ध्यान मिला है क्योंकि कुछ भविष्यवाणियाँ इसे स्टेबिलिटी के द्वीप में रखती हैं। यह एक नए g-ब्लॉक के तत्वों का पहला तत्व भी हो सकता है।
अनबीउनियम अभी तक बनाया नहीं गया है। यह अनुमान लगाया जाता है कि यह वर्तमान तकनीक के साथ बनाए जा सकने वाले अंतिम कुछ तत्वों में से एक होगा; यह सीमा तत्व 120 और 124 के बीच कहीं हो सकती है। यह 119 और 120 की तुलना में बनाना और अधिक कठिन होगा। जापान के RIKEN और रूस के JINR के टीम ने भविष्य में तत्व 121 के संश्लेषण का प्रयास करने की योजना बनाई है जब वे तत्व 119 और 120 के संश्लेषण का प्रयास कर लेंगे।
अनबीउनियम का आवर्त सारणी में स्थान lanthanum और actinium के समान गुणों का सुझाव देता है; हालांकि, रिलेटिविस्टिक प्रभाव इसके कुछ गुणों को पारंपरिक रूप से अपेक्षित गुणों से अलग कर सकते हैं। उदाहरण के लिए, अनबीउनियम की संभावना एक s2p वैलेंस इलेक्ट्रॉन कॉन्फ़िगरेशन होने की है, जिसे lanthanum और actinium के s2d या मैडेलंग नियम से अपेक्षित s2g से अलग किया जाता है, लेकिन यह इसके रसायन में कोई बड़ा प्रभाव नहीं डालेगा। यह इसकी पहली आयनीकरण ऊर्जा को आवर्त रुझानों से अपेक्षित से काफी कम कर देगा।
संश्लेषण का इतिहास[सम्पादन]

Fusion reactions producing superheavy elements can be divided into "hot" and "cold" fusion, depending on the excitation energy of the compound nucleus produced. In hot fusion reactions, very light, high-energy projectiles are accelerated toward very heavy targets (actinides), giving rise to compound nuclei at high excitation energies (~40–50 MeV) that may fission or evaporate several (3 to 5) neutrons.[२] In cold fusion reactions (which use heavier projectiles, typically from the fourth period, and lighter targets, usually lead and bismuth), the fused nuclei produced have a relatively low excitation energy (~10–20 MeV), which decreases the probability that these products will undergo fission reactions. As the fused nuclei cool to the ground state, they require emission of only one or two neutrons. However, hot fusion reactions tend to produce more neutron-rich products because the actinides have the highest neutron-to-proton ratios of any element that can presently be made in macroscopic quantities; it is currently the only method to produce the superheavy elements from flerovium (element 114) onward.[३]
Attempts to synthesize elements 119 and 120 push the limits of current technology, due to the decreasing cross sections of the production reactions and their probably short half-lives, expected to be on the order of microseconds.[४] Heavier elements, beginning with element 121, would likely be too short-lived to be detected with current technology, decaying within a microsecond before reaching the detectors. Where this one-microsecond border of half-lives lies is not known, and this may allow the synthesis of some isotopes of elements 121 through 124, with the exact limit depending on the model chosen for predicting nuclide masses.[५] It is also possible that element 120 is the last element reachable with current experimental techniques, and that elements from 121 onward will require new methods.[४]
पिछले संश्लेषण प्रयास[सम्पादन]
अनबीउनियम के संश्लेषण का पहला प्रयास 1977 में Gesellschaft für Schwerionenforschung (GSI) में Darmstadt, Germany में किया गया था, जहाँ uranium-238 पर copper-65 आयनों से बॉम्बार्डमेंट किया गया था:
- साँचा:Nuclide + साँचा:Nuclide → साँचा:Nuclide* → no atoms
कोई परमाणु पहचाने नहीं गए.[६]
भविष्य के संश्लेषण के लिए संभावनाएँ[सम्पादन]
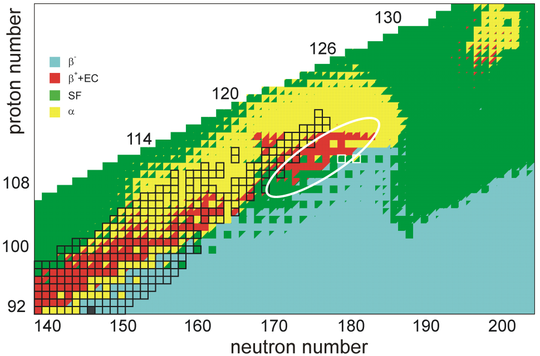
Currently, the beam intensities at superheavy element facilities result in about 1012 projectiles hitting the target per second; this cannot be increased without burning the target and the detector, and producing larger amounts of the increasingly unstable actinides needed for the target is impractical. The team at the Joint Institute for Nuclear Research (JINR) in Dubna has built a new superheavy element factory (SHE-factory) with improved detectors and the ability to work on a smaller scale, but even so, continuing beyond element 120 and perhaps 121 would be a great challenge.[८] It is possible that the age of fusion–evaporation reactions to produce new superheavy elements is coming to an end due to the increasingly short half-lives to spontaneous fission and the looming proton nuclear drip line, so that new techniques such as nuclear transfer reactions (for example, firing uranium nuclei at each other and letting them exchange protons, potentially producing products with around 120 protons) would be required to reach the superactinides.[८]
Because the cross sections of these fusion-evaporation reactions increase with the asymmetry of the reaction, titanium would be a better projectile than chromium for the synthesis of element 121, though this necessitates an einsteinium target. This poses severe challenges due to the significant heating and damage of the target due to the high radioactivity of einsteinium-254, but it would nonetheless probably be the most promising approach. It would require working on a smaller scale due to the lower amount of 254Es that can be produced. This small-scale work could in the near future only be carried out in Dubna's SHE-factory.[९]
The isotopes 299Ubu, 300Ubu, and 301Ubu, that could be produced in the reaction between 254Es and 50Ti via the 3n and 4n channels, are expected to be the only reachable unbiunium isotopes with half-lives long enough for detection. The cross sections would nonetheless push the limits of what can currently be detected. For example, in a 2016 publication, the cross section of the aforementioned reaction between 254Es and 50Ti was predicted to be around 7 fb in the 4n channel, four times lower than the lowest measured cross section for a successful reaction. A 2021 calculation gives similarly low theoretical cross sections of 10 fb for the 3n channel and 0.6 fb for the 4n channel of this reaction, along with cross sections on the order of 1–10 fb for the reactions 249Bk+54Cr, 252Es+50Ti, and 258Md+48Ca.[१०] However, 252Es and 258Md cannot currently be synthesized in sufficient quantities to form target material.[९]
Should the synthesis of unbiunium isotopes in such a reaction be successful, the resulting nuclei would decay through isotopes of ununennium that could be produced by cross-bombardments in the 248Cm+51V or 249Bk+50Ti reactions, down through known isotopes of tennessine and moscovium synthesized in the 249Bk+48Ca and 243Am+48Ca reactions.[४] The multiplicity of excited states populated by the alpha decay of odd nuclei may however preclude clear cross-bombardment cases, as was seen in the controversial link between 293Ts and 289Mc.[११][१२] Heavier isotopes are expected to be more stable; 320Ubu is predicted to be the most stable unbiunium isotope, but there is no way to synthesize it with current technology as no combination of usable target and projectile could provide enough neutrons.[१३]
The teams at RIKEN and at JINR have listed the synthesis of element 121 among their future plans.[९][१४][१५] These two laboratories are best suited to these experiments as they are the only ones in the world where long beam times are accessible for reactions with such low predicted cross-sections.[१६]
नामकरण[सम्पादन]
Using Mendeleev's nomenclature for unnamed and undiscovered elements, unbiunium should be known as eka-actinium. Using the 1979 IUPAC recommendations, the element should be temporarily called unbiunium (symbol Ubu) until it is discovered, the discovery is confirmed, and a permanent name chosen.[१७] Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations are mostly ignored among scientists who work theoretically or experimentally on superheavy elements, who call it "element 121", with the symbol E121, (121), or 121.[१८]
नाभिकीय स्थिरता और समस्थानिक[सम्पादन]
The stability of nuclei decreases greatly with the increase in atomic number after curium, element 96, whose half-life is four orders of magnitude longer than that of any currently known higher-numbered element. All isotopes with an atomic number above 101 undergo radioactive decay with half-lives of less than 30 hours. No elements with atomic numbers above 82 (after lead) have stable isotopes.[१९] Nevertheless, for reasons not yet well understood, there is a slight increase of nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg and stemming from the stabilizing effects of the closed nuclear shells around Z = 114 (or possibly 120, 122, 124, or 126) and N = 184 (and possibly also N = 228), explains why superheavy elements last longer than predicted.[२०][२१] In fact, the very existence of elements heavier than rutherfordium can be attested to shell effects and the island of stability, as spontaneous fission would rapidly cause such nuclei to disintegrate in a model neglecting such factors.[२२]
A 2016 calculation of the half-lives of the isotopes of unbiunium from 290Ubu to 339Ubu suggested that those from 290Ubu to 303Ubu would not be bound and would decay through proton emission, those from 304Ubu through 314Ubu would undergo alpha decay, and those from 315Ubu to 339Ubu would undergo spontaneous fission. Only the isotopes from 309Ubu to 314Ubu would have long enough alpha-decay lifetimes to be detected in laboratories, starting decay chains terminating in spontaneous fission at moscovium, tennessine, or ununennium. This would present a grave problem for experiments aiming at synthesizing isotopes of unbiunium if true, because the isotopes whose alpha decay could be observed could not be reached by any presently usable combination of target and projectile.[२३] Calculations in 2016 and 2017 by the same authors on elements 123 and 125 suggest a less bleak outcome, with alpha decay chains from the more reachable nuclides 300–307Ubt passing through unbiunium and leading down to bohrium or nihonium.[२४] It has also been suggested that cluster decay might be a significant decay mode in competition with alpha decay and spontaneous fission in the region past Z = 120, which would pose yet another hurdle for experimental identification of these nuclides.[२५][२६][२७]
अनुमानित रसायन[सम्पादन]
अनबीउनियम को अपनी एक अनूठी रूप से लंबी संक्रमण शृंखला का पहला तत्व माना जाता है, जिसे superactinides कहा जाता है actinides के समानांतर। हालाँकि इसका व्यवहार lanthanum और actinium से काफी अलग नहीं होना चाहिए, यह आवर्त सारणी के नियम के अनुप्रयोग के लिए एक सीमा स्थापित कर सकता है; तत्व 121 से, 5g, 6f, 7d, और 8p1/2 ऑर्बिटल्स अपनी बहुत ही करीबी ऊर्जाओं के कारण एक साथ भरे जाने की संभावना है, और तत्व 150s और 160s के पास, 9s, 9p1/2, और 8p3/2 उप-शेल्स शामिल हो जाते हैं, जिसका मतलब है कि तत्व 121 और 122 के बाद (जिसके लिए पूर्ण गणनाएँ की गई हैं वह अंतिम तत्व है) के रसायन इतना समान होगा कि उनका आवर्त सारणी में स्थान एक औपचारिक बात होगा।[२८][१८]
Aufbau principle के आधार पर, अनबीउनियम परमाणु पर 5g उप-शेल भरना शुरू होना चाहिए। हालाँकि, जबकि lanthanum में 4f
- ↑ Zagrebaev, Karpov & Greiner 2013.
- ↑ Barber, Robert C.; Gäggeler, Heinz W.; Karol, Paul J. एवम् अन्य (2009). "Discovery of the element with atomic number 112 (IUPAC Technical Report)". Pure and Applied Chemistry 81 (7): 1331. doi:10.1351/PAC-REP-08-03-05. http://doc.rero.ch/record/297412/files/pac-rep-08-03-05.pdf.
- ↑ Armbruster, Peter; Munzenberg, Gottfried (1989). "Creating superheavy elements". Scientific American 34: 36–42.
- ↑ ४.० ४.१ ४.२ Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?". Journal of Physics: Conference Series 420 (1): 012001. arXiv:1207.5700. Bibcode 2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. http://nrv.jinr.ru/pdf_file/J_phys_2013.pdf.
- ↑ ५.० ५.१ Karpov, Alexander; Zagrebaev, Valery; Greiner, Walter (1 April 2015). "Superheavy Nuclei: which regions of nuclear map are accessible in the nearest studies". Texas A & M University. http://cyclotron.tamu.edu/she2015/assets/pdfs/presentations/Karpov_SHE_2015_TAMU.pdf.
- ↑ Hofmann, Sigurd (2002). On Beyond Uranium. Taylor & Francis. प॰ 105. आई॰ऍस॰बी॰ऍन॰ 978-0-415-28496-7. https://archive.org/details/onbeyonduraniumj0000hofm/page/105.
- ↑ Greiner, Walter (2013). "Nuclei: superheavy–superneutronic–strange–and of antimatter". Journal of Physics: Conference Series 413 (1): 012002. Bibcode 2013JPhCS.413a2002G. doi:10.1088/1742-6596/420/1/012002. http://inspirehep.net/record/1221632/files/jpconf13_413_012002.pdf. अभिगमन तिथि: 30 April 2017.
- ↑ ८.० ८.१ Krämer, Katrina (29 January 2016). "Beyond element 118: the next row of the periodic table". Chemistry World. https://www.chemistryworld.com/news/beyond-element-118-the-next-row-of-the-periodic-table/9400.article.
- ↑ ९.० ९.१ ९.२ Roberto, J. B. (31 March 2015). "Actinide Targets for Super-Heavy Element Research". Texas A & M University. http://cyclotron.tamu.edu/she2015/assets/pdfs/presentations/Roberto_SHE_2015_TAMU.pdf.
- ↑ Safoora, V.; Santhosh, K. P. (2021). "Synthesis of superheavy element Z=121". DAE Symposium on Nuclear Physics. pp. 205–206. http://www.sympnp.org/proceedings/.
- ↑ Forsberg, U.; Rudolph, D.; Fahlander, C. एवम् अन्य (9 July 2016). "A new assessment of the alleged link between element 115 and element 117 decay chains". Physics Letters B 760 (2016): 293–296. Bibcode 2016PhLB..760..293F. doi:10.1016/j.physletb.2016.07.008. http://portal.research.lu.se/portal/files/9762047/PhysLettB760_293_2016.pdf. अभिगमन तिथि: 2 April 2016.
- ↑ Forsberg, Ulrika; Fahlander, Claes; Rudolph, Dirk (2016). "Congruence of decay chains of elements 113, 115, and 117". Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. doi:10.1051/epjconf/201613102003. http://www.epj-conferences.org/articles/epjconf/pdf/2016/26/epjconf-NS160-02003.pdf.
- ↑ सन्दर्भ त्रुटि: अमान्य
<ref>टैग;Amadorनामक संदर्भ की जानकारी नहीं है - ↑ Morita, Kōsuke (5 February 2016). "The Discovery of Element 113". https://www.youtube.com/watch?v=kGVkkVMgvOg.
- ↑ Sokolova, Svetlana; Popeko, Andrei (24 May 2021). "How are new chemical elements born?". JINR. http://www.jinr.ru/posts/how-are-new-chemical-elements-born/. "JINR is currently building the first factory of superheavy elements in the world to synthesize elements 119, 120 and 121, and to study in depth the properties of previously obtained elements."
- ↑ Hagino, Kouichi; Hofmann, Sigurd; Miyatake, Hiroari; Nakahara, Hiromichi (July 2012). "平成23年度 研究業績レビュー(中間レビュー)の実施について" (ja में). RIKEN. http://www.riken.jp/~/media/riken/about/reports/evaluation/rnc/rep/rnc-morita2012-report-e.pdf.
- ↑ Chatt, J. (1979). "Recommendations for the naming of elements of atomic numbers greater than 100". Pure and Applied Chemistry 51 (2): 381–384. doi:10.1351/pac197951020381.
- ↑ १८.० १८.१ सन्दर्भ त्रुटि: अमान्य
<ref>टैग;Haireनामक संदर्भ की जानकारी नहीं है - ↑ de Marcillac, Pierre; Coron, Noël; Dambier, Gérard एवम् अन्य (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature 422 (6934): 876–878. Bibcode 2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
- ↑ Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9th सं॰). Wiley-Interscience. आई॰ऍस॰बी॰ऍन॰ 978-0-471-33230-5. OCLC 223349096.
- ↑ Koura, H.; Chiba, S. (2013). "Single-Particle Levels of Spherical Nuclei in the Superheavy and Extremely Superheavy Mass Region". Journal of the Physical Society of Japan 82 (1): 014201. Bibcode 2013JPSJ...82a4201K. doi:10.7566/JPSJ.82.014201. https://www.researchgate.net/publication/258799250.
- ↑ Möller, P. (2016). "The limits of the nuclear chart set by fission and alpha decay". EPJ Web of Conferences 131: 03002:1–8. Bibcode 2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002. http://inspirehep.net/record/1502715/files/epjconf-NS160-03002.pdf.
- ↑ Santhosh, K. P.; Nithya, C. (27 September 2016). "Predictions on the alpha decay chains of superheavy nuclei with Z = 121 within the range 290 ≤ A ≤ 339". International Journal of Modern Physics E 25 (10): 1650079. arXiv:1609.05495. Bibcode 2016IJMPE..2550079S. doi:10.1142/S0218301316500798.
- ↑ Santhosh, K. P.; Nithya, C. (28 December 2016). "Theoretical predictions on the decay properties of superheavy nuclei Z = 123 in the region 297 ≤ A ≤ 307". The European Physical Journal A 52 (371): 371. Bibcode 2016EPJA...52..371S. doi:10.1140/epja/i2016-16371-y.
- ↑ Santhosh, K. P.; Sukumaran, Indu (25 January 2017). "Decay of heavy particles from Z = 125 superheavy nuclei in the region A = 295–325 using different versions of proximity potential". International Journal of Modern Physics E 26 (3): 1750003. Bibcode 2017IJMPE..2650003S. doi:10.1142/S0218301317500033.
- ↑ Poenaru, Dorin N.; Gherghescu, R. A.; Greiner, W.; Shakib, Nafiseh (September 2014). "How Rare Is Cluster Decay of Superheavy Nuclei?". Nuclear Physics: Present and Future FIAS Interdisciplinary Science Series 2015. doi:10.1007/978-3-319-10199-6_13.
- ↑ Poenaru, Dorin N.; Gherghescu, R. A.; Greiner, W. (March 2012). "Cluster decay of superheavy nuclei". Physical Review C 85 (3): 034615. Bibcode 2012PhRvC..85c4615P. doi:10.1103/PhysRevC.85.034615. https://www.researchgate.net/publication/235507943. अभिगमन तिथि: 2 May 2017.
- ↑ Loveland, Walter (2015). "The Quest for Superheavy Elements". 2015 National Nuclear Physics Summer School. http://www.int.washington.edu/NNPSS/2015/nnpss2015_SHE_Loveland.pdf.
